Somatosensory Evoked Potentials (SSEP): Difference between revisions
| (39 intermediate revisions by 2 users not shown) | |||
| Line 17: | Line 17: | ||
#'''Constant Current vs Constant Voltage''': Constant current stimulation is recommended for consistent and reliable stimulation for optimal SSEP recording in the OR, especially for long surgical procedures. Constant current stimuli may compensate for any changes in electrical conductivity, or electrode contact resistance. | #'''Constant Current vs Constant Voltage''': Constant current stimulation is recommended for consistent and reliable stimulation for optimal SSEP recording in the OR, especially for long surgical procedures. Constant current stimuli may compensate for any changes in electrical conductivity, or electrode contact resistance. | ||
#'''Stimulation Parameters''': a series of rectangular pulses with certain pulse width and frequency are typically used as electrical stimuli for SSEP recording. | #'''Stimulation Parameters''': a series of rectangular pulses with certain pulse width and frequency are typically used as electrical stimuli for SSEP recording. | ||
##Pulse width (or pulse duration): 200-300 microsecond is suggested | ##'''Pulse width (or pulse duration)''': 200-300 microsecond is suggested | ||
##Frequency (or rep rate): Higher stimulus rate is desired for quick acquisition of SSEP responses in the operating room. However increasing the rate too high may result in degradation of the responses; also it is limited when | ##'''Frequency (or rep rate)''': Higher stimulus rate is desired for quick acquisition of SSEP responses in the operating room. However increasing the rate too high may result in degradation of the responses; also it is limited when interleaving stimulation of 2 or 4 limbs. In general a frequency between 2 and 5 Hz are recommended. To avoid synchronization between the responses and the underlying electrical noise, most commonly 60Hz or 50Hz in different countries, the stimulus rate should not be a submultiple of the noise frequency. Sometimes a slight change of stimulus rate, for example from 4.80 to 4.13, may improve the quality of the evoked responses. | ||
##Intensity | ##'''Intensity''': Supramaximal stimulation should be used to produce repeatable responses. Some factors, such as pathology of the peripheral nerves, large or edematous extremities, distance of the electrodes to the underlying nerves, types of stimulating electrodes, may limit the effectiveness of stimulation. A stimulus of 50mA or greater is required sometimes. There is concern of tissue damage from high current densities at the stimulation site which is rare. | ||
== Recording Techniques == | == Recording Techniques == | ||
== | #'''Electrodes''': Metal surface cup electrodes are non-invasive electrodes, which can be applied with conductive gel after skin preparation. Subdermal needles are also commonly used in the operating room; some are available with angled or hooked tips. A corkscrew version can be screwed into the scalp for prolonged recording, however excessive bleeding can be a concern. For direct cortical mapping and recording, a strip or grid electrode array can be used for cranial surgeries. | ||
#'''Recording Sites''' | |||
##Cortical recording of upper extremity SSEP: It is the post-central gyrus of somatosensory cortex, contralateral to the stimulated limb. The locations are called CP3 and CP4 which are 2 cm posterior to the C3 and C4 positions of the 10-20 International System of EEG electrode placement. The recording montage can be CP3-Fz or CP3-CP4 for right arm stimulation; CP4-Fz or CP4-CP3 for left arm stimulation. | |||
##Cortical recording of lower extremity SSEP: CPz is the active electrode site, which is 2 cm posterior to Cz. The traditional derivation of recording is CPz-Fz; however it should not be the only standard recording derivation, according to studies for optimized lower extremity SSEP recording by MacDonald DB et al and many others. Often time CPz-CPc (which is CPz-CP4 for left leg stimulation, and CPz-CP3 for right) may produce higher amplitude and more reliable signals. | |||
##Subcortical: The recording can be made at posterior cervical spine, one or linked earlobes, or mastoid. Subcortical responses are less affected by inhalational agents. However, the subcortical SSEP response is normally very well defined for upper extremity stimulation, it is often poorly defined for lower extremity stimulation. | |||
##Peripheral nerve: Usually it is the ipsilateral Erb's point for upper extremity stimulation, and ipsilateral popliteal fossa for lower extremity stimulation. This is done to verify the status of the peripheral stimulation. | |||
#'''Averaging:''' The SSEP amplitudes tend to be low; as high as only several microvolts, or as low as less than a microvolts especially with pathological subjects. Averaging is required to record the signal against biological and ambient noise. SSEP signal is time-locked to the stimulus and most of the noise occurs randomly, allowing the noise to be averaged out with averaging of repeated responses. Some earlier guidelines such as that from American EEG Society suggested acquiring 500-2000 trails per averaged response. However for surgical monitoring well defined evoked potentials should be acquired as quickly as possible. With good preparation of the recording sites to reduce impedance, and optimized recording montages, clean signals could be recorded as few as 50-200 trials. | |||
#'''Recording Parameters''' | |||
##Filters: More environmental noise in the operating room and necessity of quick acquisition make it important to choose optimal filter settings, different from laboratory SSEP diagnostic studies. The majority of the energy contained in cortical SSEP is present in the frequency bandpass above 30Hz and below 500Hz; filters can be set to (10-30) Hz to (250-1000) Hz. The relative frequency content of the subcortical or peripheral responses is much higher, thus the filters can be set to (30-100)Hz to (500-2000) Hz. | |||
##Timebase: The timebase is usually set at 50 milliseconds for upper extremity SSEPs, and 100 milliseconds for upper extremity SSEPs. It is based on the normal conduction time between the stimulation and recording sites. It may need adjustment depending on the age and size of the individual, and any pathological conditions. | |||
##Sensitivity: The median amplitude of SSEP is about 1 microvolt. The recording sensitivity could range from 0.1 to 5 microvolts/unit. With direct cortical recording during cranial surgeries, the amplitude can be high and the sensitivity my be set to 20-50 microvolts/unit. | |||
##Sweep delay: Normally it should be avoided to use sweep delay. However it may be necessary when large stimulus artifact is present. | |||
##Interleaving recording: Alternating recording of SSEP responses to stimulation of 2 or 4 limbs at the same time is available with modern recording devices from different manufacturers. | |||
== Waveforms == | |||
'''Upper Extremity SSEPs''' | |||
#N9: Recorded from ipsilateral Erb's point, and sometimes it's called EP. It is a compound action potential from the axons to the median nerve or ulnar nerve stimulation. | |||
#N13-P14: Cervical potentials, recorded near C5 spinal process. The origin is thought to be dorsal horn neurons, and the caudal medial lemniscus. | |||
#N20-P23: Scalp potentials, generated from the thalamocortical radiations. | |||
'''Lower Extremity SSEPs''' | |||
#LP: Recorded from the T12 spinal process, arise from the afferent nerve volley in the dorsal roots and dorsal root entry zone. | |||
#N34: Recorded at the cervical spine; the amplitude can be very low and difficult to identify. The origin is thought to be brainstem or perhaps thalamus. | |||
#P37-N45: Scalp potentials, reflect activation of the primary somatosensory cortex. | |||
[[Image:SSEP Waveforms.jpg|center|2000px|750px]] | |||
== Intraoperative Monitoring == | == Intraoperative Monitoring == | ||
The basic principle of intraoperative monitoring with mixed nerve SSEP is to stimulate nerves distal to the surgical site, and to record responses proximal to the surgical site. In most cases, these recording sites should include one cortical and one subcortical recording site. | |||
#'''Data acquisition''': Baseline SSEP responses should be established, ideally after induction and before patient positioning and surgical incision. Data should be collected throughout the surgical procedure, with corresponding documentation of surgical events such as incision, exposure, decompression, instrumentation, closure, etc. In addition, relevant physiological variables such as blood pressure and temperature changes, anesthetic agents being used and the levels, significant signal changes during the surgery and any communication and interventions should be documented. | |||
#'''Alert criteria''': Significant signal changes, with 50% decrease of peak to peak amplitude and 10% increase of latency, may be indication of nervous system functional changes and should be reported to the operating surgeon, and call for possible intervention. | |||
Depending on the specific surgery, planning is required to monitor the part of the nervous system at risk. | |||
#'''Peripheral nerve and plexus.''' | |||
As mentioned above, the lower extremity somatosensory evoked potentials are typically monitored via the posterior tibial nerve. The tibial nerve is a major component of the sciatic nerve and originates from the L4-S3 spinal nerve roots. The tibial nerve provides sensory information from the posterolateral leg, lateral foot and the bottom of the foot. | |||
Saphenous nerve SSEPs are useful for monitoring the femoral nerve during transpsoas (lateral) lumbar fusions (Silverstein et al., 2014; Spine). The femoral nerve and lumbar plexus are at risk for injury with the transpsoas approach because of the lateral angle of the approach and because of nerve compression from the retractors. The femoral nerve is the major branch of the lumbar plexus and originates from the L2-4 spinal nerve roots; and the saphenous nerve is a sensory branch of the femoral nerve and innervates the medial side of the leg and foot. The saphenous nerve is not superficial and is surrounded by the vastus medialis and sartorius on the medial part of the thigh. Therefore, it is advised to use needle electrodes for deeper stimulation through the tissue. Higher current intensities and pulse durations relative to posterior tibial nerve SSEPs may be required for a good signal to noise ratio. The cortical responses are likely to be shorter in latency compared to posterior tibial nerve SSEPs. | |||
Superficial peroneal nerve SSEPs have been used to monitor lower lumbar nerve root dysfunction during L4-5 lumbar decompressions (Yue and Martinez, Spine J.). The superficial peroneal nerve is one of two branches of the common peroneal nerve and originates from the L4-S1 spinal nerve roots. The superficial peroneal nerve provides sensory innervation from the anterolateral aspect of the lower leg and foot. Yue and Martinez showed that superficial peroneal nerve SSEPs were more sensitive than posterior tibial nerve SSEPs at detecting L4-5 nerve root dysfunction. | |||
#'''Spinal nerve root''' | |||
#'''Spinal cord''' | |||
#'''Brainstem and thalamus''' | |||
#'''Brain''' | |||
== Anesthesia | == Systemic and Anesthesia Factors == | ||
#'''Temperature'''. The temperature of the patient can increase or decrease SSEP latencies but does not necessarily affect amplitudes. | |||
#'''Blood pressure'''. An intraoperative decline in blood pressure is associated with a loss in SSEP signals. | |||
#'''Halogenated inhalational agents'''. Higher levels of gas anesthesia (> Half MAC) can increase SSEP latencies and reduce SSEP amplitudes. | |||
#'''Nitrous oxide'''. Nitrous oxide decreases SSEP amplitudes but has no effect on latencies. | |||
#'''Intravenous analgesic agents'''. Intravenous anesthetics, such as propofol, do significantly influence SSEP latencies or amplitudes. | |||
#'''Muscle relaxants'''. Neuromuscular blockers do not influence SSEP latencies or amplitudes. | |||
==References== | ==References== | ||
# Brown RH, Nash CL, Berilla JA, Amaddio MD. Cortical evoked potential monitoring. A system for intraoperative monitoring of spinal cord function. Spine 1984; 9: 256-261. | |||
# Celesia GG. Somatosensory evoked potentials recorded directly from human thalamus and Sm I cortical area. Arch Neurol 1979; 36: 399-405. | |||
# Cohen AR, Young W, Ransohoff J. Intraspinal localization of the somatosensory evoked potential. Neurosurgery 1981; 9: 157-62. | |||
# Kelly DL Jr, Goldring S, O’Leary JL. Averaged evoked somatosensory responses from exposed cortex of man. Arch Neurol 1965; 13: 1-9. | |||
# Larson SJ, Sances A. Evoked potentials in man: neurosurgical applications. Am J Surg 1966; 111: 857-861. | # Larson SJ, Sances A. Evoked potentials in man: neurosurgical applications. Am J Surg 1966; 111: 857-861. | ||
# MacDonald DB. Individually optimizing posterior tibial somatosensory evoked potential P37 scalp derivations for intraoperative monitoring. J Clin Neurophysiol. 2001 Jul;18(4):364-71. | |||
# Mauguiere F. Anatomic origin of the cervical N13 potential evoked by upper extremity stimulation. J of Clin Neurophysiology 2000; 17: 236-245. | |||
# McCallum JE, Bennett MH. Electrophysiologic monitoring of spinal cord function during intraspinal surgery. Surg Forum 1975; 26: 469-471. | # McCallum JE, Bennett MH. Electrophysiologic monitoring of spinal cord function during intraspinal surgery. Surg Forum 1975; 26: 469-471. | ||
# Nash CL, Long RA, Schatzinger LA, Brown RH. Spinal cord monitoring during operative treatment of the spine. Clin Orthop 1977; 126: 100-105. | # Nash CL, Long RA, Schatzinger LA, Brown RH. Spinal cord monitoring during operative treatment of the spine. Clin Orthop 1977; 126: 100-105. | ||
# Nuwer MR, Dawson EG, Carlson LG, Kanim LEA, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencepholog and Clin Neurophysiol 1995; 96: 6-11. | |||
# Nuwer MR, et al. IFCN recommended standards for short latency somatosensory evoked potentials. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology 1994; 91: 6-11. | |||
# Powers SK, Bolger CA, Edwards MSB. Spinal cord pathways mediating somatosensory evoked potentials. J Neurosurg 1982; 57: 472-82. | # Powers SK, Bolger CA, Edwards MSB. Spinal cord pathways mediating somatosensory evoked potentials. J Neurosurg 1982; 57: 472-82. | ||
# Simpson RK JR, Blackburn JG, Martin HF III, Katz S. Peripheral nerve fibers and spinal cord pathway contribution to the somatosensory evoked potentials. Exp Neurol 1981; 73: 700-15. | # Simpson RK JR, Blackburn JG, Martin HF III, Katz S. Peripheral nerve fibers and spinal cord pathway contribution to the somatosensory evoked potentials. Exp Neurol 1981; 73: 700-15. | ||
# Sloan TB. Anesthetic Effects on Electrophysiologic Recordings. Journal of Clinical Neurophysiology 1998; 15: 217-226. | |||
# Toleikis JR. Intraoperative monitoring using somatosensory evoked potentials: A position statement by the American Society of Neurophysiological Monitoring. J Clin Monit Comput. 2005; 19(3):241-58. | # Toleikis JR. Intraoperative monitoring using somatosensory evoked potentials: A position statement by the American Society of Neurophysiological Monitoring. J Clin Monit Comput. 2005; 19(3):241-58. | ||
# Wang BP, Turner LA, Kamp AM, Venier LH. CPz-CP4 and CPz-CP3 are superior to CPz-FPz for recording left and right tibial nerve somatosensory evoked potentials for intraoperative monitoring. A review study in 264 patients. 2006 The 17th Annual Meeting of American Society of Neurophysiological Monitoring. | |||
# Yanni DS, Ulkatan S, Deletis V, Barrenechea IJ, Sen C, Perin NI. Utility of neurophysiological monitoring using dorsal column mapping in intramedullary spinal cord surgery. J Neurosurg Spine. 2010;12:623-628. | |||
# York DH, Chabot RJ, Gaines RW. Response variability of somatosensory evoked potentials during scoliosis surgery. Spine 1987; 12: 864-876. | |||
Latest revision as of 20:47, 3 October 2024
Somatosensory evoked potentials (SSEP) are recorded from the central nervous system following stimulation of peripheral nerves.
Introduction
Somatosensory Evoked Potentials (SSEPs) are electric signals recorded from the scalp or spine following stimulation to the peripheral nerves. They are time-locked responses, representing the function of the ascending sensory pathways. Early in the 1960s Larson et al introduced the use of somatosensory evoked potentials to monitor neural structure during neurosurgical procedures. It was utilized as a supplement to the wake-up test during correctional spinal surgeries for spinal deformities such as scoliosis to provide warning of compromised spinal cord function to the spine surgeons, as reported by McCallum et al and Nash et al in the 1970s. Since then SSEP has become one of the earliest and primary tools for intraoperative neurophysiological monitoring.
Somatosensory Pathways
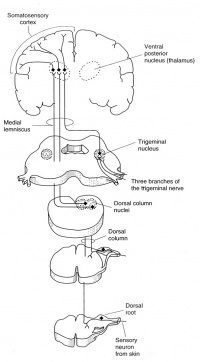
Distal peripheral nerves are stimulated for SSEP recordings; typically the median or ulnar nerve at the wrist for upper extremity SSEPs, or posterior tibial nerve at the ankle for lower extremity SSEPs. The ascending sensory volley enters the spinal cord through dorsal nerve roots. Multiple dorsal or posterior column spinal pathways such as the gracile fasciculus (legs and trunk) and the cuneate fasciculus (arms and trunk) mediate the SSEP responses. They arrive at the medulla and make synaptic connection at the medullary nuclei (nucleus cuneatus and nucleus gracilis). From there they cross and ascend in the medial lemniscal pathways to thalamic nuclei, which in turn project up to the somatosenory cortex.
There is no synapses between the peripheral stimulation sites and the medullary nuclei. Synapses are the sites of action for inhalational anesthetic agents. Therefore any SSEP responses recorded below the level of medullary nuclei are affected only minimally by general anesthesia. The cortical SSEP responses, however, are greatly affected by inhalational agents; whichever recorded anesthetic management is very much required.
Stimulation
- Stimulation Sites: SSEPs may be elicited by electrical stimulation to major nerve trunks or dermatomes. Upper extremity mixed or major nerve SSEPs are typically obtained by stimulating the median nerve or ulnar nerve near the wrist; or sometimes the ulnar nerve at the elbow. Lower extremity SSEPs are normally recorded to stimulation of the posterior tibial nerve at the ankle, or peroneal nerve near the head of the fibula at the knee. Usually the anode electrode should be placed 2-4 cm distal to the cathode electrode to avoid anodal block.
- Electrodes: Subdermal needle electrodes or adhesive surface electrodes are most commonly used for intraoperative SSEP recordings. Some other types of electrodes such as bar electrodes and EEG metal disc electrodes are more suitable for diagnostic SSEP recordings in the lab. Needle electrodes can be placed closer to the underlying nerves than surface electrodes; however they are associated with additional risks of infection, bleeding, and inadvertent needle sticks.
- Constant Current vs Constant Voltage: Constant current stimulation is recommended for consistent and reliable stimulation for optimal SSEP recording in the OR, especially for long surgical procedures. Constant current stimuli may compensate for any changes in electrical conductivity, or electrode contact resistance.
- Stimulation Parameters: a series of rectangular pulses with certain pulse width and frequency are typically used as electrical stimuli for SSEP recording.
- Pulse width (or pulse duration): 200-300 microsecond is suggested
- Frequency (or rep rate): Higher stimulus rate is desired for quick acquisition of SSEP responses in the operating room. However increasing the rate too high may result in degradation of the responses; also it is limited when interleaving stimulation of 2 or 4 limbs. In general a frequency between 2 and 5 Hz are recommended. To avoid synchronization between the responses and the underlying electrical noise, most commonly 60Hz or 50Hz in different countries, the stimulus rate should not be a submultiple of the noise frequency. Sometimes a slight change of stimulus rate, for example from 4.80 to 4.13, may improve the quality of the evoked responses.
- Intensity: Supramaximal stimulation should be used to produce repeatable responses. Some factors, such as pathology of the peripheral nerves, large or edematous extremities, distance of the electrodes to the underlying nerves, types of stimulating electrodes, may limit the effectiveness of stimulation. A stimulus of 50mA or greater is required sometimes. There is concern of tissue damage from high current densities at the stimulation site which is rare.
Recording Techniques
- Electrodes: Metal surface cup electrodes are non-invasive electrodes, which can be applied with conductive gel after skin preparation. Subdermal needles are also commonly used in the operating room; some are available with angled or hooked tips. A corkscrew version can be screwed into the scalp for prolonged recording, however excessive bleeding can be a concern. For direct cortical mapping and recording, a strip or grid electrode array can be used for cranial surgeries.
- Recording Sites
- Cortical recording of upper extremity SSEP: It is the post-central gyrus of somatosensory cortex, contralateral to the stimulated limb. The locations are called CP3 and CP4 which are 2 cm posterior to the C3 and C4 positions of the 10-20 International System of EEG electrode placement. The recording montage can be CP3-Fz or CP3-CP4 for right arm stimulation; CP4-Fz or CP4-CP3 for left arm stimulation.
- Cortical recording of lower extremity SSEP: CPz is the active electrode site, which is 2 cm posterior to Cz. The traditional derivation of recording is CPz-Fz; however it should not be the only standard recording derivation, according to studies for optimized lower extremity SSEP recording by MacDonald DB et al and many others. Often time CPz-CPc (which is CPz-CP4 for left leg stimulation, and CPz-CP3 for right) may produce higher amplitude and more reliable signals.
- Subcortical: The recording can be made at posterior cervical spine, one or linked earlobes, or mastoid. Subcortical responses are less affected by inhalational agents. However, the subcortical SSEP response is normally very well defined for upper extremity stimulation, it is often poorly defined for lower extremity stimulation.
- Peripheral nerve: Usually it is the ipsilateral Erb's point for upper extremity stimulation, and ipsilateral popliteal fossa for lower extremity stimulation. This is done to verify the status of the peripheral stimulation.
- Averaging: The SSEP amplitudes tend to be low; as high as only several microvolts, or as low as less than a microvolts especially with pathological subjects. Averaging is required to record the signal against biological and ambient noise. SSEP signal is time-locked to the stimulus and most of the noise occurs randomly, allowing the noise to be averaged out with averaging of repeated responses. Some earlier guidelines such as that from American EEG Society suggested acquiring 500-2000 trails per averaged response. However for surgical monitoring well defined evoked potentials should be acquired as quickly as possible. With good preparation of the recording sites to reduce impedance, and optimized recording montages, clean signals could be recorded as few as 50-200 trials.
- Recording Parameters
- Filters: More environmental noise in the operating room and necessity of quick acquisition make it important to choose optimal filter settings, different from laboratory SSEP diagnostic studies. The majority of the energy contained in cortical SSEP is present in the frequency bandpass above 30Hz and below 500Hz; filters can be set to (10-30) Hz to (250-1000) Hz. The relative frequency content of the subcortical or peripheral responses is much higher, thus the filters can be set to (30-100)Hz to (500-2000) Hz.
- Timebase: The timebase is usually set at 50 milliseconds for upper extremity SSEPs, and 100 milliseconds for upper extremity SSEPs. It is based on the normal conduction time between the stimulation and recording sites. It may need adjustment depending on the age and size of the individual, and any pathological conditions.
- Sensitivity: The median amplitude of SSEP is about 1 microvolt. The recording sensitivity could range from 0.1 to 5 microvolts/unit. With direct cortical recording during cranial surgeries, the amplitude can be high and the sensitivity my be set to 20-50 microvolts/unit.
- Sweep delay: Normally it should be avoided to use sweep delay. However it may be necessary when large stimulus artifact is present.
- Interleaving recording: Alternating recording of SSEP responses to stimulation of 2 or 4 limbs at the same time is available with modern recording devices from different manufacturers.
Waveforms
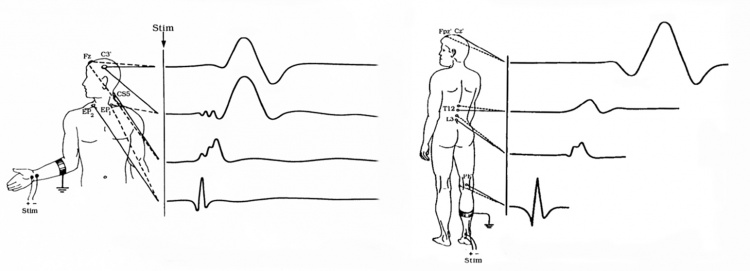
Upper Extremity SSEPs
- N9: Recorded from ipsilateral Erb's point, and sometimes it's called EP. It is a compound action potential from the axons to the median nerve or ulnar nerve stimulation.
- N13-P14: Cervical potentials, recorded near C5 spinal process. The origin is thought to be dorsal horn neurons, and the caudal medial lemniscus.
- N20-P23: Scalp potentials, generated from the thalamocortical radiations.
Lower Extremity SSEPs
- LP: Recorded from the T12 spinal process, arise from the afferent nerve volley in the dorsal roots and dorsal root entry zone.
- N34: Recorded at the cervical spine; the amplitude can be very low and difficult to identify. The origin is thought to be brainstem or perhaps thalamus.
- P37-N45: Scalp potentials, reflect activation of the primary somatosensory cortex.
Intraoperative Monitoring
The basic principle of intraoperative monitoring with mixed nerve SSEP is to stimulate nerves distal to the surgical site, and to record responses proximal to the surgical site. In most cases, these recording sites should include one cortical and one subcortical recording site.
- Data acquisition: Baseline SSEP responses should be established, ideally after induction and before patient positioning and surgical incision. Data should be collected throughout the surgical procedure, with corresponding documentation of surgical events such as incision, exposure, decompression, instrumentation, closure, etc. In addition, relevant physiological variables such as blood pressure and temperature changes, anesthetic agents being used and the levels, significant signal changes during the surgery and any communication and interventions should be documented.
- Alert criteria: Significant signal changes, with 50% decrease of peak to peak amplitude and 10% increase of latency, may be indication of nervous system functional changes and should be reported to the operating surgeon, and call for possible intervention.
Depending on the specific surgery, planning is required to monitor the part of the nervous system at risk.
- Peripheral nerve and plexus.
As mentioned above, the lower extremity somatosensory evoked potentials are typically monitored via the posterior tibial nerve. The tibial nerve is a major component of the sciatic nerve and originates from the L4-S3 spinal nerve roots. The tibial nerve provides sensory information from the posterolateral leg, lateral foot and the bottom of the foot.
Saphenous nerve SSEPs are useful for monitoring the femoral nerve during transpsoas (lateral) lumbar fusions (Silverstein et al., 2014; Spine). The femoral nerve and lumbar plexus are at risk for injury with the transpsoas approach because of the lateral angle of the approach and because of nerve compression from the retractors. The femoral nerve is the major branch of the lumbar plexus and originates from the L2-4 spinal nerve roots; and the saphenous nerve is a sensory branch of the femoral nerve and innervates the medial side of the leg and foot. The saphenous nerve is not superficial and is surrounded by the vastus medialis and sartorius on the medial part of the thigh. Therefore, it is advised to use needle electrodes for deeper stimulation through the tissue. Higher current intensities and pulse durations relative to posterior tibial nerve SSEPs may be required for a good signal to noise ratio. The cortical responses are likely to be shorter in latency compared to posterior tibial nerve SSEPs.
Superficial peroneal nerve SSEPs have been used to monitor lower lumbar nerve root dysfunction during L4-5 lumbar decompressions (Yue and Martinez, Spine J.). The superficial peroneal nerve is one of two branches of the common peroneal nerve and originates from the L4-S1 spinal nerve roots. The superficial peroneal nerve provides sensory innervation from the anterolateral aspect of the lower leg and foot. Yue and Martinez showed that superficial peroneal nerve SSEPs were more sensitive than posterior tibial nerve SSEPs at detecting L4-5 nerve root dysfunction.
- Spinal nerve root
- Spinal cord
- Brainstem and thalamus
- Brain
Systemic and Anesthesia Factors
- Temperature. The temperature of the patient can increase or decrease SSEP latencies but does not necessarily affect amplitudes.
- Blood pressure. An intraoperative decline in blood pressure is associated with a loss in SSEP signals.
- Halogenated inhalational agents. Higher levels of gas anesthesia (> Half MAC) can increase SSEP latencies and reduce SSEP amplitudes.
- Nitrous oxide. Nitrous oxide decreases SSEP amplitudes but has no effect on latencies.
- Intravenous analgesic agents. Intravenous anesthetics, such as propofol, do significantly influence SSEP latencies or amplitudes.
- Muscle relaxants. Neuromuscular blockers do not influence SSEP latencies or amplitudes.
References
- Brown RH, Nash CL, Berilla JA, Amaddio MD. Cortical evoked potential monitoring. A system for intraoperative monitoring of spinal cord function. Spine 1984; 9: 256-261.
- Celesia GG. Somatosensory evoked potentials recorded directly from human thalamus and Sm I cortical area. Arch Neurol 1979; 36: 399-405.
- Cohen AR, Young W, Ransohoff J. Intraspinal localization of the somatosensory evoked potential. Neurosurgery 1981; 9: 157-62.
- Kelly DL Jr, Goldring S, O’Leary JL. Averaged evoked somatosensory responses from exposed cortex of man. Arch Neurol 1965; 13: 1-9.
- Larson SJ, Sances A. Evoked potentials in man: neurosurgical applications. Am J Surg 1966; 111: 857-861.
- MacDonald DB. Individually optimizing posterior tibial somatosensory evoked potential P37 scalp derivations for intraoperative monitoring. J Clin Neurophysiol. 2001 Jul;18(4):364-71.
- Mauguiere F. Anatomic origin of the cervical N13 potential evoked by upper extremity stimulation. J of Clin Neurophysiology 2000; 17: 236-245.
- McCallum JE, Bennett MH. Electrophysiologic monitoring of spinal cord function during intraspinal surgery. Surg Forum 1975; 26: 469-471.
- Nash CL, Long RA, Schatzinger LA, Brown RH. Spinal cord monitoring during operative treatment of the spine. Clin Orthop 1977; 126: 100-105.
- Nuwer MR, Dawson EG, Carlson LG, Kanim LEA, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencepholog and Clin Neurophysiol 1995; 96: 6-11.
- Nuwer MR, et al. IFCN recommended standards for short latency somatosensory evoked potentials. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology 1994; 91: 6-11.
- Powers SK, Bolger CA, Edwards MSB. Spinal cord pathways mediating somatosensory evoked potentials. J Neurosurg 1982; 57: 472-82.
- Simpson RK JR, Blackburn JG, Martin HF III, Katz S. Peripheral nerve fibers and spinal cord pathway contribution to the somatosensory evoked potentials. Exp Neurol 1981; 73: 700-15.
- Sloan TB. Anesthetic Effects on Electrophysiologic Recordings. Journal of Clinical Neurophysiology 1998; 15: 217-226.
- Toleikis JR. Intraoperative monitoring using somatosensory evoked potentials: A position statement by the American Society of Neurophysiological Monitoring. J Clin Monit Comput. 2005; 19(3):241-58.
- Wang BP, Turner LA, Kamp AM, Venier LH. CPz-CP4 and CPz-CP3 are superior to CPz-FPz for recording left and right tibial nerve somatosensory evoked potentials for intraoperative monitoring. A review study in 264 patients. 2006 The 17th Annual Meeting of American Society of Neurophysiological Monitoring.
- Yanni DS, Ulkatan S, Deletis V, Barrenechea IJ, Sen C, Perin NI. Utility of neurophysiological monitoring using dorsal column mapping in intramedullary spinal cord surgery. J Neurosurg Spine. 2010;12:623-628.
- York DH, Chabot RJ, Gaines RW. Response variability of somatosensory evoked potentials during scoliosis surgery. Spine 1987; 12: 864-876.